AHCC®蘑菇复合多糖--激活免疫抗癌

AHCC®是获得最多临床研究的专有蘑菇菌丝体复合多糖,多用于预防和治疗癌症、降低化疗副作用和清除HPV感染及提高免疫等。它是美国纪念斯凯隆癌症中心推荐给医生使用的药用菌品牌。
简介
AHCC®即Active Hexose Correlated Compound(活性己糖相关化合物,中文简称“依诺金”)是专有香菇菌丝体的标准化提取物,含有香菇菌丝体液体培养获得的低聚糖和氨基酸等混合物1,2。它由日本AminoUp公司与东京大学药学院于1987年研发和推出,因其特含有部分酰化的α-葡聚糖而著称3,4。自然(Nature)出版集团官网报道指出,AHCC复合多糖可以刺激肠道中人类免疫细胞上的特定受体,这些受体可以识别病毒和细菌等外来病原体,从而支持健康的免疫应答5。AHCC®是目前世界上研究最多的专业免疫补充剂,已经获得了100多项科学研究支持,其中包括30多项人体研究。它是美国纪念斯凯隆癌症中心(MSK,全美排名第二)推荐给医生使用的仅有几种药用菌之一6。已有40多个国家和1000多家医疗机构使用AHCC来降低健康群体和免疫受损患者的感染率,改善癌症患者的预后,减少化疗副作用,帮助控制HPV感染,管理丙型肝炎患者的病毒载量,并有利于肝病患者7。
临床总结
AHCC富含葡聚糖(约74%)及特含有α-1,4键的低分子量(~5KDa)多糖(约占20%)8,被认为可增强其生物效应9,调节免疫细胞的数量和功能8;AHCC与TLR-2和TLR-4结合,充当免疫调节剂10 。患者使用AHCC可预防和治疗癌症6。AHCC显示出抗炎11和抗癌作用12-16,增强对微生物感染的抵抗力17,18,并可能预防氧化应激诱导的疾病19。在健康成年人中,AHCC改善了T细胞免疫反应20,增加了树突细胞数量和功能21,改善了对流感疫苗的抗体反应22,当与长双歧杆菌一起使用时,调节了T调节和树突细胞表型,有利于抗生素使用后的抗炎反应23。
初步研究结果表明,AHCC可以改善肝细胞癌根治性切除术后的预后并预防复发24,25,改善新辅助治疗期间的营养状况26,27,并减少化疗相关的不良反应28,29,以及帮助清除HPV感染30,31。需要进一步的研究来确定AHCC的治疗潜力。
临床研究
AHCC® 成为全球100多家知名医疗机构和著名大学医学院的研究重点,已发表30多项人体研究包括免疫、抗癌、抑制HPV和酒精性肝病等。与肿瘤相关的部分简介如下:1.减少化疗药物副作用,提高晚期癌症患者生活质量28。
20名癌症患者接受了第一个周期的化疗,然后第二个周期接受AHCC。在化疗期间,每周通过血液检测、EORTC QLQ-C30问卷和唾液中6型疱疹病毒(HHV-6)的DNA水平评估不良反应和生活质量。化疗后HHV-6的DNA水平显著升高。不过,AHCC的给药显著降低了化疗期间唾液中HHV-6的水平,不仅改善了EORTC QLQ-C30问卷中的生活质量评分,还改善了血液毒性和肝毒性。这些发现表明,唾液HHV-6水平可能是化疗期间患者生活质量的良好生物标志物,AHCC可能对癌症化疗患者的化疗相关不良反应和生活质量产生有益影响28。
其他研究包括:补充AHCC组(n=35)可以降低药物吉西他滨对胰腺癌患者的副作用29。接受铂基化疗的上皮性卵巢癌或腹膜癌患者(n=14),补充AHCC(3g/天)可显著减少恶心和呕吐,CD8+T细胞淋巴细胞水平显著升高9。
2.降低癌症治疗后的复发风险25。
一项非随机、开放和非对照研究,接受根治性肝切除术的肝癌患者(n=29)每天口服3次AHCC(1g)持续2年。结果切除术后2年无复发生存率为48%,所有有治疗史的患者无复发率为55.2%。术后第一个月,血清白蛋白水平降至最低,6个月后逐渐恢复到术前水平。随访期间,淋巴细胞百分比几乎没有变化。肝切除术后,基于炎症的预后评分保持在有利水平。在所有患者中均未观察到毒性和不良事件25。
3.AHCC可持久清除人乳头瘤病毒(HPV)30,31。
在确诊为持续性HR-HPV+感染的女性中进行了两项试点研究30,每项研究有10名患者。第一项研究评估了5周至6个月的AHCC 3g/天,第二项研究评估的AHCC 1g/天(<8个月)。每次随访时监测HR-HPV DNA状态和免疫小组。结果:6名患者中有4名(66.7%)在AHCC 3g治疗3-6个月后证实HR-HPV清除。同样,9名患者中有4名(44%)在服用1g AHCC 7个月后证实HR-HPV清除。在清除HR-HPV感染的患者中观察到IFNβ的抑制<25pg/mL30。
2022年发表相关的一项验证性II期随机、双盲、安慰剂对照研究(CTN:NCT02405533)31,受试者为2年以上的50名持续感染高危HPV女性,设计服用AHCC(3g/天)至少6个月。在研究完成时(n=41),共有34名患者(22名盲法患者和12名非盲法患者)接受了AHCC补充,总体反应率为58.8%,清除了HPV持续感染。在入组时,确诊为持续HPV感染的女性的平均IFN-β水平为60.5±37.6pg/ml。在接受AHCC补充的女性中,将IFN-β抑制到20pg/ml以下与T淋巴细胞和IFN-γ的增加以及HPV感染的持久清除有关(如图2)。结果表明,补充AHCC(3g/天)可有效支持宿主免疫系统消除持续的HPV感染,并且耐受性良好,没有报告明显的不良副作用31。
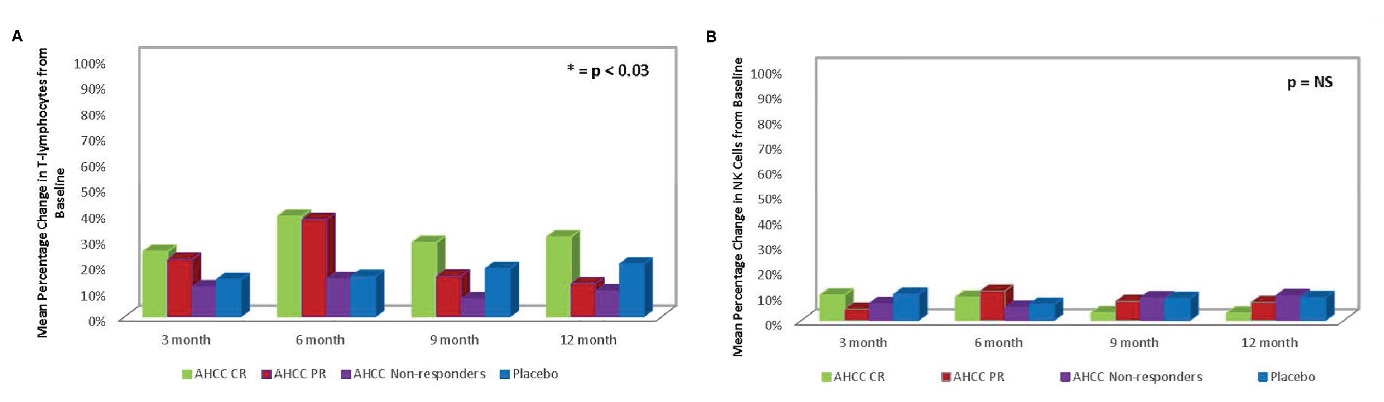
图1.(A)II期患者T淋巴细胞与基线相比的平均百分比变化总结,T淋巴细胞变化与HPV感染的清除有关;(B)II期患者干扰素-γ(IFN-γ)反应总结。IFN-γ,一种II型干扰素的增加与IFN-β,一种I型干扰素的减少相关,并最终清除HPV感染。(图源:Smith at al | Front Oncol ,2022)
参考用量
临床试验中使用的一些剂量包括以下内容:- 每天1g用于支持免疫系统,降低感染风险32。
- 酒精引起的肝酶升高,每天1-3g33。
- 肝癌患者每天服用3g25。
- 清除HPV每天3g31。
- 胰腺癌患者每天6g29。
一般建议用量:1-3g/天,推荐剂量可能因使用目的而不同8。
与药物等相互作用
- CYP450底物:AHCC诱导CYP2D6,这可能会降低底物药物如阿霉素或昂丹司琼的活性。临床意义尚不清楚34。
- 芳香酶抑制剂:AHCC诱导芳香酶,并可能降低芳香酶抑制药物(如来曲唑)的活性。临床相关性尚未确定35。
不良反应
总体上,AHCC副作用很少。- 临床试验中报告了腹泻和瘙痒36。
- 其他研究报告出现轻度烧心、胀气和疲劳等31。
产品品牌
据AminoUp官网,全球销售的所有 AHCC® 散装粉末仅在在日本札幌本部生产和供应。由于使用AHCC的全球用户越来越多,市场上出现了许多假冒AHCC产品。为此,AminoUP在其官网(www.aminoupworld.com)上公示了原料分销商和终端产品品牌商,以支持消费者购买到真实的AHCC产品。此外,AHCC协会官网(www.ahcc.net/id-tested/)也公示了北美地区销售的AHCC产品品牌商。
如何在国内购买到正宗的AHCC产品,本网站将与日本厂商协商,敬请期待。
参考文献
1. Ritz BW. Supplementation with active hexose correlated compound increases survival following infectious challenge in mice. Nutrition Reviews. 2008;66(9):526–531.
2. Sato K et al. Profile summary of AHCC: composition. In: Kulkarni A. D., Calder P. C., Ito T., editors. Clinician's Guide to AHCC. International Congress on Nutrition and Integrative Medicine; 2017. pp. 24–33.
3. Masuda Y et al. Antitumor activity of orally administered maitake α-glucan by stimulating antitumor immune response in murine tumor. PLoS One. 2017;12(3):p.e0173621.
4. Hoshi H et al. Isolation and characterization of a novel immunomodulatory alpha-glucan-protein complex from the mycelium of Tricholoma matsutake in basidiomycetes. Journal of Agricultural and Food Chemistry. 2005;53(23):8948–8956.
5. Available at. https://www.nature.com/articles/d42473-020-00414-3. Latest accessed Aug 26, 2024.
6. Available at. https://www.mskcc.org/cancer-care/integrative-medicine/herbs/ahcc. Latest accessed Aug 28, 2024
7. Available at. https://www.ahcc.net/what-is-ahcc/. Latest accessed Aug 23,2024
8. Shin MS et al. The Effects of AHCC®, a Standardized Extract of Cultured Lentinura edodes Mycelia, on Natural Killer and T Cells in Health and Disease: Reviews on Human and Animal Studies. J Immunol Res. 2019; 2019: 3758576.
9. Suknikhom W et al. The Effects of Active Hexose Correlated Compound (AHCC) on Levels of CD4+ and CD8+ in Patients with Epithelial Ovarian Cancer or Peritoneal Cancer Receiving Platinum Based Chemotherapy. Asian Pac J Cancer Prev. 2017 Mar 1;18(3):633-638.
10. Mallet JF et al. Active Hexose Correlated Compound (AHCC) promotes an intestinal immune response in BALB/c mice and in primary intestinal epithelial cell culture involving toll-like receptors TLR-2 and TLR-4. Eur J Nutr. 2016 Feb;55(1):139-46.
11. Mascaraque C et al. Active hexose correlated compound exerts therapeutic effects in lymphocyte driven colitis. Mol Nutr Food Res. 2014 Dec;58(12):2379-82.
12. Kidd PM. The use of mushroom glucans and proteoglycans in cancer treatment. Altern Med Rev. Feb 2000;5(1):4-27.
13. Yagita A et al. H-2 haplotype-dependent serum IL-12 production in tumor-bearing mice treated with various mycelial extracts. In Vivo. Jan-Feb 2002;16(1):49-54.
14. Hirose A et al. The influence of active hexose correlated compound (AHCC) on cisplatin-evoked chemotherapeutic and side effects in tumor-bearing mice. Toxicol Appl Pharmacol. Jul 15 2007;222(2):152-158.
15. Kuhara K et al. CUB Domain-containing Protein 1 (CDCP1) Is Down-regulated by Active Hexose-correlated Compound in Human Pancreatic Cancer Cells. Anticancer Res. 2018 Nov;38(11):6107-6111.
16. Suenaga S et al. Active hexose-correlated compound down-regulates HSP27 of pancreatic cancer cells, and helps the cytotoxic effect of gemcitabine. Anticancer Res. 2014 Jan;34(1):141-6.
17. Aviles H et al. Active hexose correlated compound enhances resistance to Klebsiella pneumoniae infection in mice in the hind limb-unloading model of spaceflight conditions. J Appl Physiol. Aug 2003;95(2):491-496.
18. Wang S et al. Oral Administration of Active Hexose Correlated Compound Enhances Host Resistance to West Nile Encephalitis in Mice. J Nutr. 2009;139(3):598-602.
19. Ye SF et al. Suppressive effects of Active Hexose Correlated Compound on the increased activity of hepatic and renal ornithine decarboxylase induced by oxidative stress. Life Sci. Dec 19 2003;74(5):593-602.
20. Yin Z et al. Effects of active hexose correlated compound on frequency of CD4+ and CD8+ T cells producing interferon-γ and/or tumor necrosis factor-α in healthy adults. Hum Immunol. 2010;71(12):1187-90.
21. Terakawa N et al. Immunological effect of active hexose correlated compound (AHCC) in healthy volunteers: a double-blind, placebo-controlled trial. Nutr Cancer. 2008;60(5):643-651.
22. Roman BE et al. Short-term supplementation with active hexose correlated compound improves the antibody response to influenza B vaccine. Nutr Res. 2013 Jan;33(1):12-7.
23. Chowdhury AH et al. Modulation of T Regulatory and Dendritic Cell Phenotypes Following Ingestion of Bifidobacterium longum, AHCC® and Azithromycin in Healthy Individuals. Nutrients. 2019 Oct 15;11(10). pii: E2470.
24. Matsui Yet al. Improved prognosis of postoperative hepatocellular carcinoma patients when treated with functional foods: a prospective cohort study. J Hepatol. Jul 2002;37(1):78-86.
25. Kamiyama T et al. Preventing recurrence of hepatocellular carcinoma after curative hepatectomy with active hexose correlated compound derived from Lentinula edodes mycelia. Integr Cancer Ther. 2022 Jan-Dec:21:15347354211073066.
26. Hashimoto D et al. Nutritional impact of active hexose-correlated compound for patients with resectable or borderline-resectable pancreatic cancer treated with neoadjuvant therapy. Surg Today. 2021 Nov;51(11):1872-1876.
27. Yanagimoto H et al. Efficacy of Lentinula edodes Mycelia Extract on Chemotherapy-Related Tasted Disorders in Pancreatic Cancer Patients. Nutr Cancer. 2023;75(1):236-246.
28. Ito T et al. Reduction of adverse effects by a mushroom product, active hexose correlated compound (AHCC) in patients with advanced cancer during chemotherapy—the significance of the levels of HHV-6 DNA in saliva as a surrogate biomarker during chemotherapy. Nutr Cancer. 2014;66(3):377-382.
29. Yanagimoto H et al. Alleviating effect of active hexose correlated compound (AHCC) on chemotherapy-related adverse events in patients with unresectable pancreatic ductal adenocarcinoma. Nutr Cancer. 2016;68(2):234-240.
30. Smith JA et al. From Bench to Bedside: Evaluation of AHCC Supplementation to Modulate the Host Immunity to Clear High-Risk Human Papillomavirus Infections. Front Oncol. 2019; 9: 173.
31. Smith JA et al. AHCC® supplementation to support immune function to clear persistent human papillomavirus infections. Front Oncol. 2022;12:881902.
32. Takanari J et al. Effects of active hexose correlated compound on the seasonal variations of immune competence in healthy subjects. J Evid Based Complementary Altern Med. 2015;20(1):28-34.
33. Kim H et al. Effect of active hexose correlated compound (AHCC) in alcohol-induced liver enzyme elevation. J Nutr Sci Vitaminol (Tokyo). 2014;60(5):348-356.
34. Mach CM et al. Evaluation of active hexose correlated compound hepatic metabolism and potential for drug interactions with chemotherapy agents. J Soc Integr Oncol. Summer 2008;6(3):105-109.
35. Mathew L et al. Evaluation of Active Hexose Correlated Compound (AHCC) in Combination With Anticancer Hormones in Orthotopic Breast Cancer Models. Integr Cancer Ther. 2017 Apr 1:1534735417704948.
36. Sumiyoshi Y et al. Dietary administration of mushroom mycelium extracts in patients with early stage prostate cancers managed expectantly: a phase II study. Jpn J Clin Oncol. 2010 Oct;40(10):967-72.
37. Hyodo I et al. Nationwide survey on complementary and alternative medicine in cancer patients in Japan. J Clin Oncol. 2005 Apr 20;23(12):2645-54.
其他参考来源
美国纪念斯凯隆癌症中心
https://www.mskcc.org/
AHCC厂商AminoUP官网
https://www.aminoup.jp/en/research/
美国国立癌症研究所
https://www.cancer.gov/
美国国立医学图书馆信息中心Pubmed
https://pubmed.ncbi.nlm.nih.gov/
来源:本网编辑 2024.09.16.

