新冠后遗症
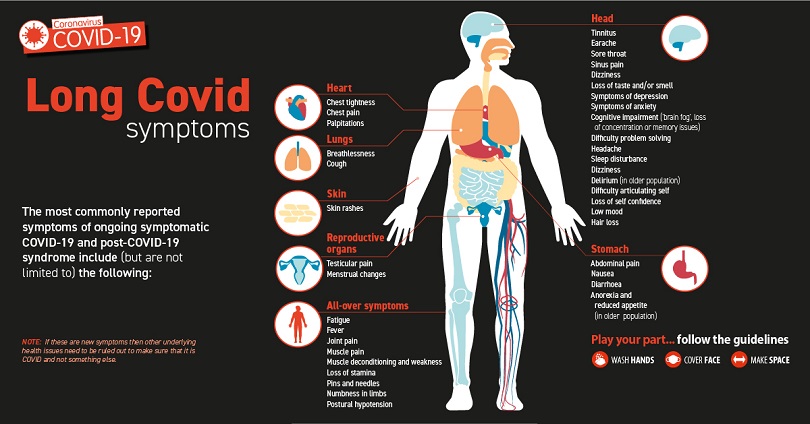
新冠后遗症涉及患者在感染新冠病毒后四个多星期出现的各种新的、复发的或持续的症状。在某些人中,新冠后遗症会持续数月或数年,甚至导致残疾。
英文名称:Long COVID,Post-COVID conditions,Post-COVID-19 syndrome, Long-haul COVID-19
定义
新冠后遗症涉及患者在感染新冠病毒后四个多星期出现的各种新的、复发的或持续的症状。在某些人中,新冠后遗症会持续数月或数年,甚至导致残疾。研究表明,在感染COVID-19 后的一个月到一年之间:
- 年龄在18至64岁:约20%的人患有至少一种可能由新冠病毒感染引起的疾病。
- 65岁及以上的人群:25%的人至少患有一种可能由新冠病毒感染引起的疾病。
症状
患有后 COVID 病症(或长期 COVID)的人可能会出现许多症状。有时症状甚至会消失或再次出现。最常报告的症状包括:
- 疲劳或乏力
- 体力或脑力劳动后症状加重,又称“劳累后不适”
- 发热
- 肺部(呼吸系统)症状,包括咳嗽、呼吸急促或困难
- 神经系统症状或精神健康状况,包括难以思考或集中注意力(或称“脑雾”),头痛、睡眠问题、站立时头晕、针刺感、嗅觉或味觉丧失或改变,以及抑郁或焦虑
- 关节或肌肉疼痛
- 心脏症状或状况,包括胸痛和快速或剧烈的心跳
- 消化系统症状,包括腹泻和胃痛
- 血栓和血管(血管)问题,包括从腿部深静脉进入肺部并阻塞血液流向肺部的血块(肺栓塞)
- 其他症状,例如皮疹和月经周期变化
有些症状与慢性疲劳综合症(CFS)和感染后出现的其他慢性疾病引起的症状相似。CFS涉及极度疲劳,这种疲劳会随着身体或精神活动而恶化,但不会随着休息而改善。
病因
- 器官损伤可能起到一定作用。患有新冠肺炎重病的人可能会出现影响心脏、肾脏、皮肤和大脑的器官损伤。
- 炎症和免疫系统问题可能发生。目前尚不清楚这些影响会持续多久。这些影响还可能导致新的疾病发生发展,例如糖尿病或心脏病或神经系统疾病。
- 重症患者经历可能是另一个因素。出现严重症状的人通常需要在医院重症监护室接受治疗。这可能导致极度虚弱和创伤后应激障碍,这是一种由恐怖事件引发的心理健康状况。
潜在的病理生理机制:
据《Nature》、《BMJ》等最新发表的研究1,2,与 COVID-19相关的长期器官损伤是由新冠病毒感染引起的炎症和相关的免疫反应造成的。
- 胸痛和心悸等心血管症状与内皮功能障碍、微凝血和血管密度降低有关。
- 新冠后遗症与肾损伤和2型糖尿病的风险增加有关。
- 新冠后遗症的眼科症状,包括瞳孔对光的反应改变,是由角膜中小神经纤维的损失、树突细胞密度增加和视网膜微血管系统受损引起的。
- 肺灌注改变、上皮损伤和气道内空气滞留会导致持续性咳嗽和呼吸困难等呼吸系统症状。
- 认知和神经症状,包括记忆力减退、认知能力下降、睡眠困难、感觉异常、平衡困难、对噪音和光敏感、耳鸣以及味觉和/或嗅觉丧失。潜在的病理生理机制包括犬尿氨酸通路激活、内皮损伤、凝血障碍、皮质醇水平降低、髓磷脂丢失、小胶质细胞再激活、氧化应激、缺氧和四氢生物蝶呤缺乏症。
- 腹部疼痛、恶心、食欲不振、便秘和胃灼热等胃肠道症状与普通拟杆菌和瘤胃球菌计数升高以及普拉梭菌计数降低有关。
- 与呼吸道和胃肠道症状相比,神经系统症状通常起病较晚、随时间恶化且持续时间更长,新冠后遗症在儿童和成人中的表现相似。
风险因素
新冠后遗症在成人中似乎也比在儿童和青少年中更常见,而且女性患病率高于男性。但是,任何感染新冠病毒的人都可能产生长期影响,包括没有症状或轻度感染COVID-19的人。如果出现以下情况,可能更有可能患上新冠后遗症:
- 患有严重的新冠感染疾病,尤其是当住院或需要重症监护时。
- 在感染新冠病毒之前患有基础疾病。
- 在感染新冠病毒期间或之后患有影响器官和组织的疾病(多系统炎症综合征)。
有些人在感染COVID-19后会出现新的健康状况。有些人,尤其是那些患有重症新冠病毒病的人,会在发病后出现持续数周、数月甚至数年的多器官效应或自身免疫疾病症状。多器官效应可能涉及许多身体系统,包括心脏、肺、肾脏、皮肤和大脑。由于这些影响,与未感染COVID-19的人相比,感染过新冠病毒的人更有可能患上新的疾病,例如糖尿病、心脏病、血栓或神经系统疾病。
疗法
新冠后遗症病因十分复杂、症状表现因人而异,目前医学上仅限于对症治疗。因此,应更多采取饮食和营养干预措施,以纠正患者病理生理状态,防止症状持续或恶化,从根本上恢复患者健康。调整饮食与生活方式
- 饮食方面:食用富含抗炎和改善免疫的天然食物,例如地中海饮食,包括:丰富的水果、蔬菜、豆类和全麦类;优质蛋白质,如鱼、瘦肉、家禽、鸡蛋和低脂奶酪,以及不饱和脂肪酸来源,如:特级初榨橄榄油,或坚果类等。
- 减少高糖指数食物摄入,如精制米面食品等。
- 限制食用:糖类、甜品饮料等。
- 摄入充足水分(30ml/公斤体重):包括饮水、汤料、牛奶、茶和果汁等。
- 良好睡眠和休息,不宜劳累(包括体力、脑力)过度。
- 不饮酒或适量饮酒。
- 戒烟或不吸烟。
营养与草本综合干预
主要的营养干预路径和营养素可参考如下:
1. 改善氧化应激:
一般采用的营养素包括:N乙酰半胱氨酸3,4、谷胱甘肽5,6、辅酶Q107,8、N乙酰半胱氨酸(GlyNAC)9,10,11、维生素抗氧化剂12等;
植物多酚,例如:碧萝芷(碧容健)1,13、白藜芦醇14,15、绿茶提取物16等。
2. 抗炎、维持炎症与免疫平衡:
关键营养素包括:欧米伽-3/ 鱼油17,18、N乙酰半胱氨酸19,20、姜黄素21,22、肌醇23、十六酰胺乙醇(PEA)24,25、槲皮素26等。
3. 纠正免疫失衡、维持正常的免疫反应:
主要采用的营养素包括:维生素D27,28、复合维矿素29,以及免疫刺激剂,例如:乳清蛋白30、动植物混合蛋白31、益生菌32,33、乳铁蛋白34等。
4. 调养身体、防止或降低新冠后遗症风险:
一般采用的营养素包括:烟酰胺核糖35,36、N乙酰半胱氨酸(甘氨酸协同)10,11、辅酶Q107、益生菌37、复合维矿素38、欧米伽3(鱼油)39等。
5. 防止心脏受损:纠正内源性氧化失衡、减少内皮功能障碍等。
N乙酰半胱氨酸(GlyNAC)9,10、碧萝芷40,41、辅酶Q107,42、鱼油43,44、谷胱甘肽45,46等。
6. 防止肌肉减少症:
主要采用营养素包括:乳清蛋白47、动植物混合蛋白48、复合维矿素49、维生素D350、肌酸51,52、羟甲基丁酸52,53等。
7. 缓解慢性疲劳、促进健康恢复:
通常使用的营养素包括:L肉碱54,55或乙酰L肉碱55,56、辅酶Q1057、复合维生素B58、维生素C59、复合矿物质60、必需脂肪酸61,62等
8. 增强肺功能、缓解慢性咳嗽症状:
主要使用的营养素包括:N乙酰半胱氨酸63,64、辅酶Q1065,66、常春藤叶67,68、L肉碱69、肌酸70、维生素抗氧化剂71等。
如需了解具体产品选择,可点击其个性化的综合干预方案如下:
1.新冠后遗症防控要略:
2. 长新冠症状缓解:
3. 不同性别、年龄段新冠后遗症防控:
3.1.男性新冠后遗症防控:
3.2. 女性新冠后遗症防控:
4. 重症新冠感染后康复:指因重症接受过医院治疗的患者。
以及参阅本网有关专文:
新冠后遗症的饮食与营养补充指南
医疗干预
如果出现新冠后遗症症状,应及时去看医生,并可能需要报告如下问题:
- 症状什么时候开始的
- 什么情况会使症状恶化
- 出现症状的频率
- 症状如何影响生活等
根据《Nature》2023.01.发表的文献1有关内容如下:
1.新冠后遗症的诊断和治疗主要基于症状:
包括体位性心动过速综合征(POTS)倾斜测试、用于检测心血管和肺损伤的磁共振成像(MRI)以及用于检测QRS复合波碎裂的心电图。唾液测试和血清学测试,包括红细胞变形、脂质谱、全血计数、D-二聚体和C-反应蛋白(CRP)评估,可用于评估免疫生物标志物水平。PCR(聚合酶链反应)分析用于新冠病毒RNA检测和定量,并进行抗体测试以评估针对新冠病毒的体液免疫应答。
2. 新冠后遗症药物治疗:
包括:静脉注射Ig治疗免疫功能障碍,低剂量纳曲酮治疗神经元炎症,β受体阻滞剂治疗POTS,抗凝剂治疗微斑形成,星状神经节阻滞治疗自主神经障碍。
其他选择包括:抗组胺药、Paxlovid组合包装、舒洛德和碧萝芷(即碧容健)。
3. 非药理学选择:
包括:认知障碍的认知起搏(Cognitive pacing)、胃肠道症状的饮食限制和POTS的盐摄入量增加。Covid-19疫苗对新冠后遗症提供了最低限度的保护,其发展取决于致病性新冠病毒变异体和接种剂量。据报道,新冠病毒奥密克戎BA.2亚变异株感染后,新冠后遗症更为常见。
预防
预防新冠后遗症的最佳方法是保护自己和他人免受感染。- 在必要时接受COVID-19检测,并在符合条件的情况下寻求COVID-19治疗。
- 其他预防措施包括:
- 避免与确诊或疑似感染COVID-19疾病的人密切接触
- 保持手部卫生,勤洗手,用肥皂和水洗手至少20秒。
主要参考来源:
世界卫生组织:
www.who.int
美国梅奥诊所:
www.mayoclinic.org
美国疾控中心:
www.cdc.gov
参考文献:
1. Davis HE et al. Long COVID: major findings, mechanisms and recommendations. Nature Reviews Microbiology Jan 13 2023
2. Crook H et al. Long covid-mechanisms, risk factors, and management. BMJ 2021; 374:n1648
3. De Flora S et al. Rationale for the use of N‐acetylcysteine in both prevention and adjuvant therapy of COVID‐19. FASEB J. 2020 Oct; 34(10): 13185–13193
4. Izquierdo-Alonso JL et al. N-acetylcysteine for prevention and treatment of COVID-19: Current state of evidence and future directions. J Infect Public Health. 2022 Dec;15(12):1477-1483.
5. Silvagno F et al. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants (Basel). 2020 Jul; 9(7): 624.
6. Labarrere CA et al. Glutathione deficiency in the pathogenesis of SARS-CoV-2 infection and its effects upon the host immune response in severe COVID-19 disease. Front. Microbiol., 06 October 2022
7 Hansen KS et al. High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: a randomized, phase 2, crossover trial. Lancet Reg Health Eur. 2023 Jan;24:100539.
8. Hargreaves IR et al. COVID-19, Coenzyme Q10 and Selenium. Adv Exp Med Biol. 2021;1327:161-168.
9. Kumar P et al. Severe Glutathione Deficiency, Oxidative Stress and Oxidant Damage in Adults Hospitalized with COVID-19: Implications for GlyNAC (Glycine and N-Acetylcysteine) Supplementation. Antioxidants (Basel). 2021 Dec 27;11(1):50.
10. Kumar P et al. Supplementing Glycine and N-Acetylcysteine (GlyNAC) in Older Adults Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Physical Function, and Aging Hallmarks: A Randomized Clinical Trial. J Gerontol A Biol Sci Med Sci. 2023 Jan 26;78(1):75-89.
11. Lizzo G et al. A Randomized Controlled Clinical Trial in Healthy Older Adults to Determine Efficacy of Glycine and N-Acetylcysteine Supplementation on Glutathione Redox Status and Oxidative Damage. Front Aging. 2022 Mar 7;3:852569.
12. Barrea L et al. Dietary Recommendations for Post-COVID-19 Syndrome. Nutrients. 2022 Mar; 14(6): 1305.
13. Jack N Losso. The Potential of Dietary Bioactive Compounds against SARS-CoV-2 and COVID-19-Induced Endothelial Dysfunction. Molecules. 2022 Mar 1;27(5):1623.
14. McGreary MR et al. Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19). Science Report 12;10978 (2022)
15. Singh S et al. Therapeutic Potential of Nutraceuticals and Dietary Supplements in the Prevention of Viral Diseases: A Review. Front Nutr. 2021 Sep 17;8:679312.
16. Mhatre S et al. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine. 2021 May;85:153286.
17. Hathaway D et al. Omega 3 Fatty Acids and COVID-19: A Comprehensive Review. Infect Chemother. 2020 Dec; 52(4): 478–495.
18. Fadiyah NN et al.Potential of Omega 3 Supplementation for Coronavirus Disease 2019 (COVID-19): A Scoping Review. Int J Gen Med. 2022; 15: 3915–3922.
19. Tenório MCDS et al. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants (Basel). 2021 Jun 16;10(6):967.
20. Sadowska AM et al. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther. 2007;20(1):9-22.
21. Vahedian-Azimi A et al. Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials. Nutrients. 2022 Jan 7;14(2):256. doi: 10.3390/nu14020256.
22. Thimmulappa RK et al. Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19. Heliyon. 2021 Feb; 7(2): e06350.
23. Bizzarri M et al. Inositol and pulmonary function. Could myo-inositol treatment downregulate inflammation and cytokine release syndrome in SARS-CoV-2? Eur Rev Med Pharmacol Sci. 2020 Mar;24(6):3426-3432.
24. Fonnesu R et al. Palmitoylethanolamide (PEA) Inhibits SARS-CoV-2 Entry by Interacting with S Protein and ACE-2 Receptor. Viruses. 2022 May 17;14(5):1080.
25. Noce A et al. Ultramicronized Palmitoylethanolamide (um-PEA): A New Possible Adjuvant Treatment in COVID-19 patients. Pharmaceuticals (Basel). 2021 Apr; 14(4): 336.
26. Biancatelli RMLC et al. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol., 2020 Jun v.11
27. Gibbons JB et al. Association between vitamin D supplementation and COVID-19 infection and mortality, Sci Rep 12, 19397 (2022)
28. Cynthia Aranow. Vitamin D and the Immune System. J Investig Med. 2011 Aug; 59(6): 881–886.
29. Speakman LL et al. Vitamins, supplements and COVID-19: a review of currently available evidence. Drugs Context. 2021; 10: 2021-6-2.
30. Gallo V et al. Antiviral properties of whey proteins and their activity against SARS-CoV-2 infection. J Funct Foods. 2022 Feb; 89: 104932.
31. Fernández-Quintela A et al. Key Aspects in Nutritional Management of COVID-19 Patients. J Clin Med. 2020 Aug 10;9(8):2589.
32. Yeoh YK et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021 Apr;70(4):698-706.
33. Tang H et al. Randomised, double-blind, placebo-controlled trial of Probiotics To Eliminate COVID-19 Transmission in Exposed Household Contacts (PROTECT-EHC): a clinical trial protocol. BMJ Open. 2021 May 5;11(5):e047069.
34. Rosa L et al. Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. J Clin Med. 2021 Sep; 10(18): 4276.
35. Altay O et al. Combined Metabolic Activators Accelerates Recovery in Mild-to-Moderate COVID-19. Adv Sci (Weinh). 2021 Sep;8(17):e2101222.
36. Zheng M et al. NAD+ in COVID-19 and viral infections. Trends Immunol. 2022 Apr; 43(4): 283–295.
37. Brahma S et al. Probiotics: A gut response to the COVID-19 pandemic but what does the evidence show? Clin Nutr ESPEN. 2022 Oct; 51: 17–27.
38. Beigmohammadi MT et al. The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial. Trials. 2021 Nov 14;22(1):802.
39. Ods.nih. Omega-3 Fatty Acids Fact Sheet for Health Professionals. Available at https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/
40. Belcaro G et al. Preventive effects of Pycnogenol® on cardiovascular risk factors (including endothelial function) and microcirculation in subjects recovering from coronavirus disease 2019 (COVID-19). Minerva Med. 2022 Apr;113(2):300-308.
41. Enseleit E et al. Effects of Pycnogenol on endothelial function in patients with stable coronary artery disease: a double-blind, randomized, placebo-controlled, cross-over study. Eur Heart J. 2012 Jul;33(13):1589-97.
42. DiNicolantonio JJ et al. Coenzyme Q10 for the treatment of heart failure: a review of the literature. Open Heart. 2015 Oct 19;2(1):e000326.
43. Khan SU et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine. 2021 Jul 8;38:100997.
44. Kar S et al. Fish Oil Supplementation & Coronary Artery Disease: Does It Help? Mo Med. 2012 Mar-Apr; 109(2): 142–145.
45. Matuz-Mares D et al. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants (Basel). 2021 Aug; 10(8): 1220.
46. Bajic VP et al. Glutathione “Redox Homeostasis” and Its Relation to Cardiovascular Disease. Oxid Med Cell Longev. 2019; 2019: 5028181.
47. Lin CC et al. Effects of adequate dietary protein with whey protein, leucine, and vitamin D supplementation on sarcopenia in older adults: An open-label, parallel-group study. Clin Nutr. 2021 Mar;40(3):1323-1329.
48. Carbone JW et al. The role of dietary plant and animal protein intakes on mitigating sarcopenia risk. Curr Opin Clin Nutr Metab Care. 2022 Nov; 25(6): 425–429.
49. Robinson S et al. Micronutrients and sarcopenia: current perspectives. Proc Nutr Soc. 2021 Aug;80(3):311-318.
50. Uchitomi R et al. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients. 2020 Oct; 12(10): 3189.
51. Candow DG et al. Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. J Clin Med. 2019 Apr; 8(4): 488.
52. Gielen E et al. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Nutr Rev. 2021 Jan 9;79(2):121-147.
53. Milan Holeček. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle. 2017 Aug;8(4):529-541.
54. Plioplys AV et al. Amantadine and L-carnitine treatment of Chronic Fatigue Syndrome. Neuropsychobiology. 1997;35(1):16-23. doi: 10.1159/000119325.
55. Li C et al. Carnitine and COVID-19 Susceptibility and Severity: A Mendelian Randomization Study Carnitine and COVID-19 Susceptibility and Severity: A Mendelian Randomization Study. Front Nutr. 2021; 8: 780205.
56. Tomassini V et al. Comparison of the effects of acetyl L-carnitine and amantadine for the treatment of fatigue in multiple sclerosis: results of a pilot, randomised, double-blind, crossover trial. J Neurol Sci. 2004 Mar 15;218(1-2):103-8.
57. Tsai IC et al. Effectiveness of Coenzyme Q10 Supplementation for Reducing Fatigue: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Pharmacol. 2022; 13: 883251.
58.Tardy AL et al. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients. 2020 Jan; 12(1): 228.
59. Vollbracht C et al. Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue. Nutrients. 2021 Mar 31;13(4):1154.
60. Bjørklund G et al.Chronic fatigue syndrome (CFS): Suggestions for a nutritional treatment in the therapeutic approach. Biomed Pharmacother. 2019 Jan;109:1000-1007.
61. Maes M et al. In chronic fatigue syndrome, the decreased levels of omega-3 poly-unsaturated fatty acids are related to lowered serum zinc and defects in T cell activation. Neuro Endocrinol Lett. 2005 Dec;26(6):745-51.
62. Behan PO et al. Effect of high doses of essential fatty acids on the postviral fatigue syndrome. Acta Neurol Scand. 1990 Sep;82(3):209-16.
63. Cazzola M et al. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev. 2015 Sep;24(137):451-61.
64. Sanguinetti CM. N-acetylcysteine in COPD: why, how, and when? Multidiscip Respir Med. 2016 Feb 3;11:8.
65. Zozina VI et al. Coenzyme Q10 in COPD: An Unexplored Opportunity? COPD. 2021 Feb;18(1):114-122.
66. Fujimoto S et al. Effects of coenzyme Q10 administration on pulmonary function and exercise performance in patients with chronic lung diseases. Clin Investig. 1993;71(8 Suppl):S162-6.
67. Barnes LA et al. The effects of Hedera helix on viral respiratory infections in humans: A rapid review. Adv Integr Med. 2020 Dec;7(4):222-226.
68. Schaefer A et al. A randomized, controlled, double-blind, multi-center trial to evaluate the efficacy and safety of a liquid containing ivy leaves dry extract (EA 575®) vs. placebo in the treatment of adults with acute cough Pharmazie. 2016 Sep 1;71(9):504-509.
69. Borghi-Silva A et al. L-carnitine as an ergogenic aid for patients with chronic obstructive pulmonary disease submitted to whole-body and respiratory muscle training programs. Braz J Med Biol Res. 2006 Apr;39(4):465-74.
70. Fuld JP et al. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax. 2005 Jul;60(7):531-7.
71. Tsiligianni IG et al. A systematic review of the role of vitamin insufficiencies and supplementation in COPD. Respir Res. 2010 Dec 6;11(1):171.
免责声明和安全信息
- 本信息(包括任何附带资料)不是为了取代医生或有关合格从业人士的建议或忠告。
- 任何人如果想要对本文涉及的药物、饮食、运动或其他生活方式的使用、或改变调整,以预防或治疗某一特定健康状况或疾病,应首先咨询医生或有关合格从业人士,并获得他/她们的许可。妊娠和哺乳妇女在使用本网站任何内容前,尤其应征求医生的意见。
- 除非另有说明,本网站所述内容仅适用于成人。
- 本网站所推荐的任何产品,消费者应该以实际的产品标签内容为准,尤其应关注重要的安全信息以及产品最新信息,包括剂量、使用方法和禁忌症等。
- 由于循证医学研究、文献及有关产品处于不断的变化中,本网站工作人员将尽力更新。
- 本网站不能保证所载文章内容、综合干预方案以及相关成分或产品述及的健康益处,也不承担任何责任。

